Our Research
Prion Biology & Immunology
Prion diseases are transmissible and strictly fatal neurodegenerative disorders for which no therapy is available. In humans, the most common form is sporadic Creutzfeldt-Jakob disease (CJD). Examples affecting animals are scrapie of sheep and goats, bovine spongiform encephalopathy (BSE) or mad cow disease and chronic wasting disease (CWD) of deer, elk and moose. Prion diseases are transmissible within and, with restrictions, even between species. Zoonotic transmission can occur, as exemplified by infection of humans with BSE, which gave rise to a new variant of CJD (vCJD) affecting mainly teenagers.
The causative agents of these disorders are prions, which are unique and fascinating infectious agents. They consist solely of a misfolded form of the endogenously expressed prion protein (PrPc) which can be refolded into the pathogenic form PrPSc upon direct interaction of the two isoforms. Unlike bacteria or viruses, prions do not require genetic information for propagation and replicate in a self-propagating manner. Nevertheless, prions can exist as strains which are thought to consist of different PrPSc conformers. In diseased individuals, PrPSc accumulates in brains which finally leads to neuronal cell death.
Currently, neither prophylaxis nor therapy are available to treat or prevent prion diseases. Our research program focuses on studying the subcellular trafficking of PrPc and PrPSc and the investigation of prion-host cell interaction in order to discover novel targets for interference with prion propagation and to get insight into mechanisms of neurodegeneration in prion diseases.
Research interests
We mainly use neuronal cell culture models of prion infection to find answers to the following questions:
- How does prion infection influence cellular pathways such as vesicle trafficking, and how does this relate to neurodegeneration?
- Can we use peptide aptamers for interference with prion propagation in vivo?
- Does the PrPc-PrPSc binding interface differ between prion strains?
- Is there a relationship between the biochemical properties of CWD prions and the remarkable prion shedding (e.g. in saliva, urine, feces) observed in cervids infected with CWD?
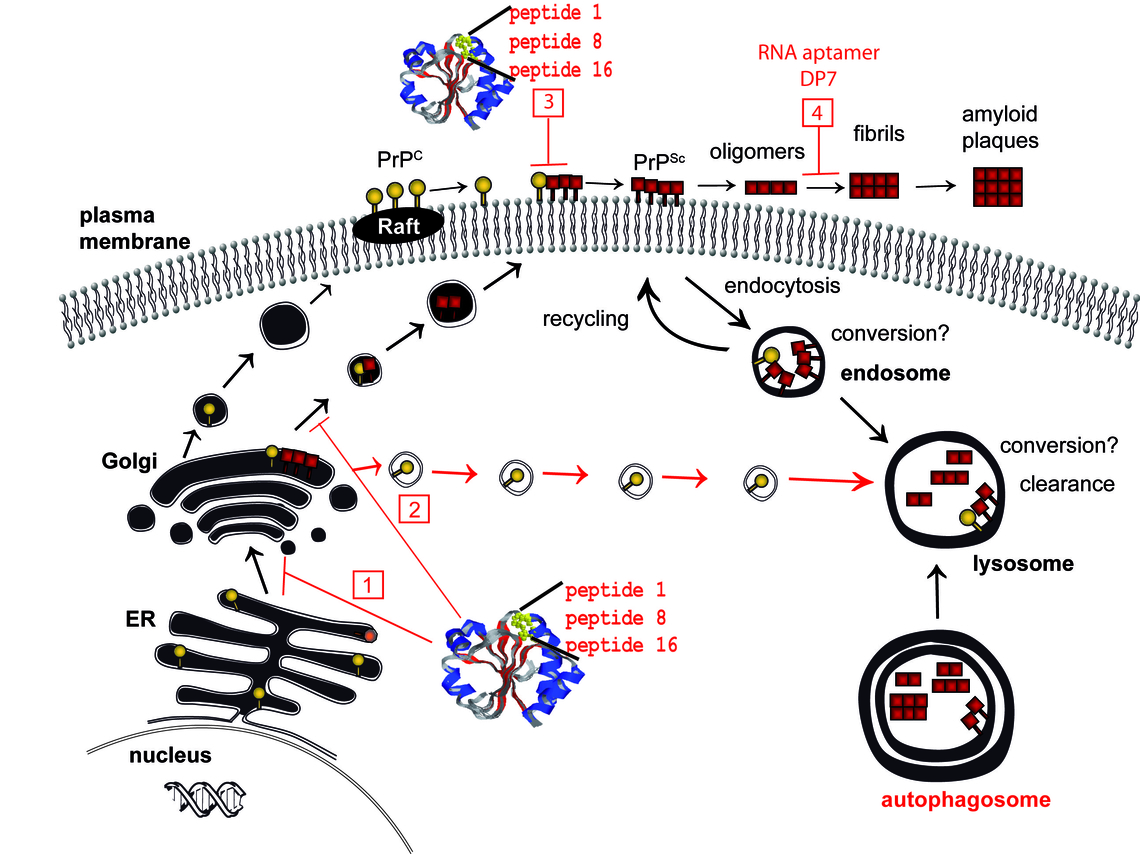
Figure. Targets for therapeutic strategies based on cell biology of prion proteins. For inhibition of the formation of PrPSc either PrPc or PrPSc can be targeted. Intracellular trapping or re-routing of PrPc can be achieved by PrP binding molecules like peptide aptamers (peptide 1, 8, 16) fused to appropriate targeting signals (1, 2; numbers referring to the numbers drawn in red boxes). Peptide aptamers expressed recombinantly in E. coli and purified can inhibit prion conversion if added to the culture media of infected cells. (3). Regarding PrPSc aggregate formation as a target, molecules like RNA aptamers that bind to PrPc (DP7) can be used to enable degradation of PrPSc (4). From Gilch & Schatzl, CMLS 2009.
Funding Sources













