Research
We use a variety of research models in our lab. Our cells-to-systems approach explores connectivity between brain regions and spinal cord circuitry as it relates to movement. Trainees will have the opportunity to learn the techniques and acquire the skills involved in using these research models.
In vitro Neonatal en bloc Spinal Cord Isolation Preparation
Here we remove the spinal cord from a neonatal mouse which allows us to sustain and record motor activity from ventral roots of the lumbar spinal cord in vitro. We also make use of a variety of other permutations of the spinal cord isolation preparation including, but not limited to, the brainstem-spinal cord and hind limb-attached preparations. We can then either electrically or chemically stimulate the spinal cord and record bouts of locomotor-like patterns of activity. In addition, these approaches can be complimented by recording intracellularly from motor neurons or interneurons via a blind patch technique. This allows us to examine activation patterns of different components of the locomotor central pattern generator network in response to a variety of neuromodulators or sensory stimuli.

Spinal Cord Slice Preparation
In many instances we wish to examine specific cells that form parts of spinal cord locomotor network. To do this we often use special genetically engineered mice that tag certain cells with a green or yellow fluorescent protein. Using a spinal cord slice approach this allows us to perform visually-guided whole-cell patch clamp techniques on these genetically identified neuronal subtypes that form parts of the locomotor central pattern generator network.

Calcium Imaging
In addition to obtaining electrophysiological recordings in the spinal cord isolation preparation we also make routine use of calcium imaging techniques. An advantage of this approach is that we have can examine the activity of populations of neurons in response to our experimental manipulations. We do this by injecting calcium dyes into specific cells that fluoresce in the presence of increased levels of calcium inside the cells, giving us an index of neuronal activity. Take a closer look at calcium imagine below which shows altered activation levels of populations of neurons in the dorsal horn of the spinal cord in response to capsaicin, a TRPV1 agonist, that is a potent activator or neurons responsible for detection of heat.

In vivo Preparations
We have advanced setups that permit studying and recording locomotion from a variety of in vivo preparations in the adult mouse. We currently utilize an adult decerebrate mouse preparation to study the effects of various neuromodulators or how sensory input affects locomotion across the developmental spectrum. We are one of two labs in the world that make routine use of this preparation and compliment it with pharmacologic and transgenic manipulations.
We also make use of in vivo optogenetic approaches that allow us to study descending control of locomotion in freely behaving mice. With this approach we make use of genetically engineered mice that express light-sensitive proteins in specific populations of neurons in the central nervous system letting us activate or silence them with light.

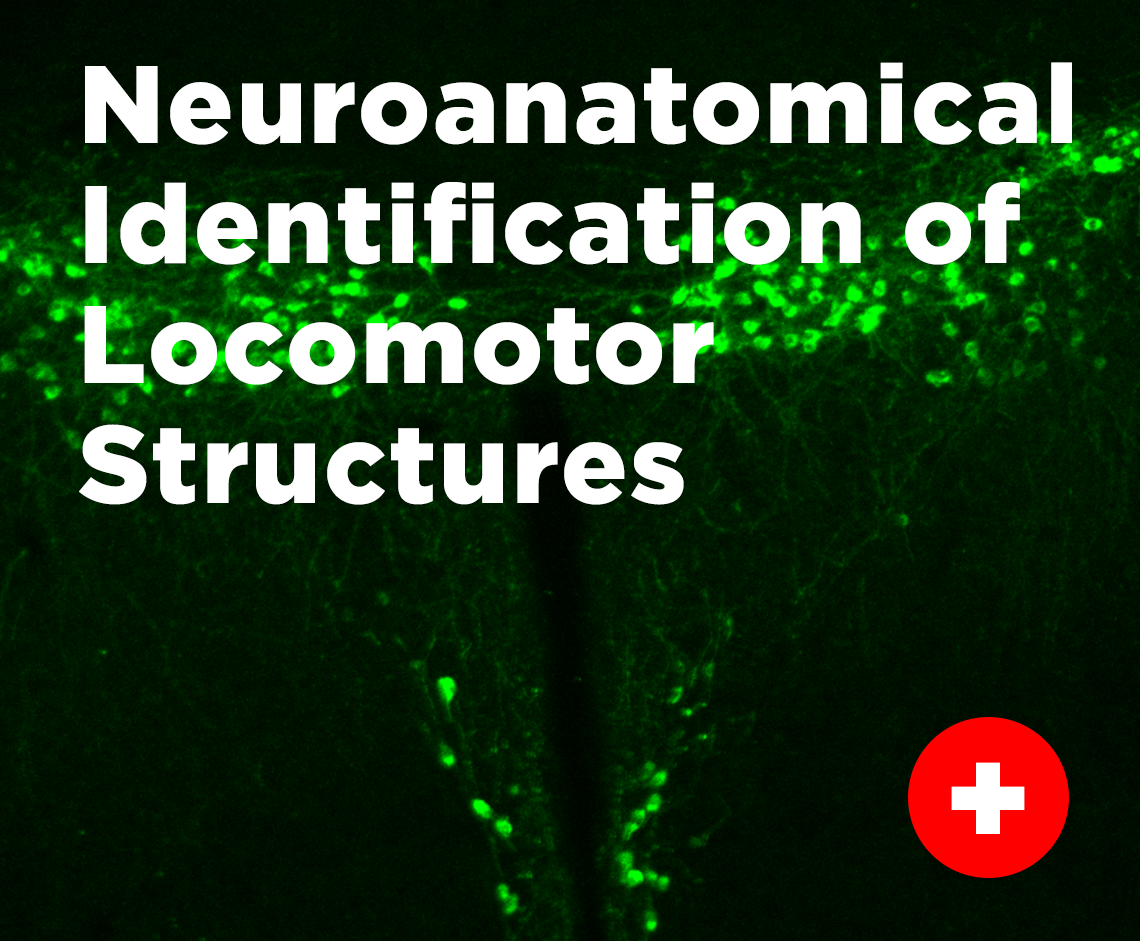
Neuroanatomical Identification of Locomotor Structures
We make use of transgenic mice and viral vectors to fluorescently label specific populations of neurons in the brain and spinal cord to characterize and study the connectivity with other regions that may play an important role in locomotor function. These neurons can be imaged using epifluorescent and confocal microscopy and will allow us to dissect and better understand the connectivity of descending centers in the brain and brain stem to different components of the locomotor central pattern generator network that is distributed through the spinal cord. This form of investigation provides valuable information that will guide future studies aimed toward better understanding the function of how these regions control locomotion.

Optogenetics and Photometry
Optogenetics is used to manipulate and study the function of neurons in various brain regions. It is a widely used technique when mapping the brain and dissecting circuits. Target cells are genetically modified to express light-sensitive proteins called opsins, such as channelrhodopsins (activated by blue light) and halorhodopsins (activated by yellow light). Light is delivered to the tissue via fibre optic cables and the opsins are activated to either stimulate or inhibit neuronal activity, and the resulting behavioural responses are recorded. This allows us to selectively activate or inhibit specific cells or neural circuits in real-time.
Photometry refers to a technique used to measure and monitor the activity of neurons. Target cells are also genetically modified to express genetically encoded calcium indicators (GECIs), (eg. GCaMP), or neurotransmitter indicators (eg. dLight). Light activates these indicators, causing these proteins to emit fluorescence when either calcium ions or neurotransmitters bind to them, indicating neuronal activity during behavioural testing.